
Bioavailability studies
View comparative bioavailability studies under fed and fasting conditions.
Single Oral Dose (1 x 18 mg) Comparative Bioavailability
Single oral dose (1 x 18 mg) comparative bioavailability study under fed conditions
Mean methylphenidate concentration-time profiles after administration of the test formulation (treatment A) and the reference product (treatment B)1,2,†
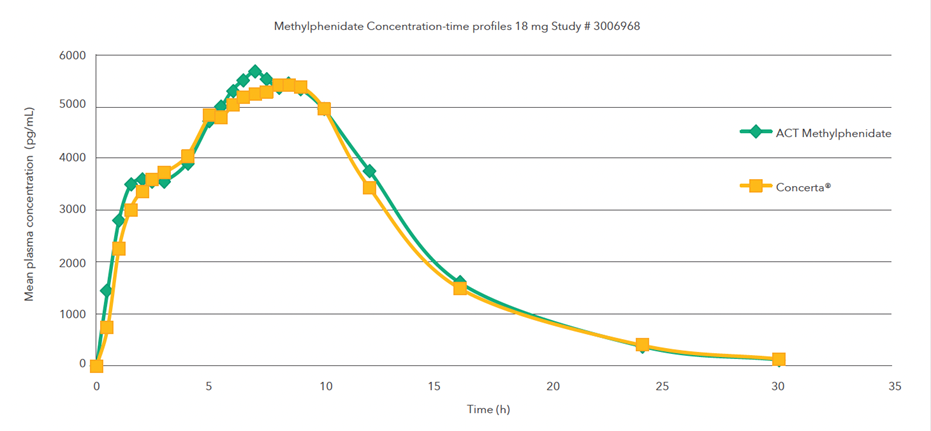
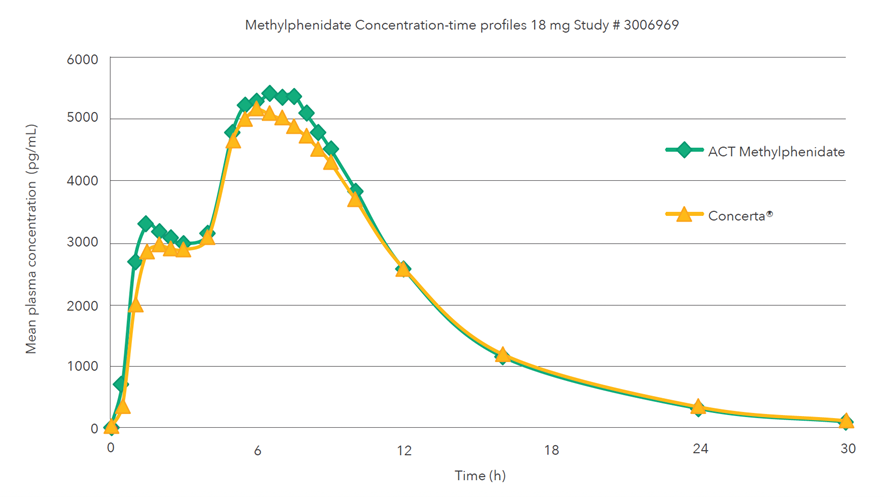
Single oral dose (1 x 18 mg) comparative bioavailability study under fasting conditions
Mean methylphenidate concentration-time profiles after administration of the test formulation (treatment A) and the reference product (treatment B)1,3,‡
†A single-dose, two-period, two-treatment, two-way crossover bioequivalence study of methylphenidate hydrochloride 18 mg extended-release tablets (Teva Canada Limited) and Concerta® (methylphenidate hydrochloride) 18 mg extended-release tablets by Janssen Inc., Canada was conducted in healthy adult subjects under fed conditions.
‡A single-dose, two-period, two-treatment, two-way crossover bioequivalence study of methylphenidate hydrochloride 18 mg extended-release tablets (Teva Canada Limited) and Concerta® (methylphenidate hydrochloride) 18 mg extended-release tablets by Janssen Inc., Canada was conducted in healthy adult subjects under fasting conditions.
Single oral dose (1 x 54 mg) comparative bioavailability study
Single oral dose (1 x 54 mg) comparative bioavailability study under fed conditions
Mean methylphenidate concentration-time profiles after administration of the test formulation (treatment A) and the reference product (treatment B)1,2,§
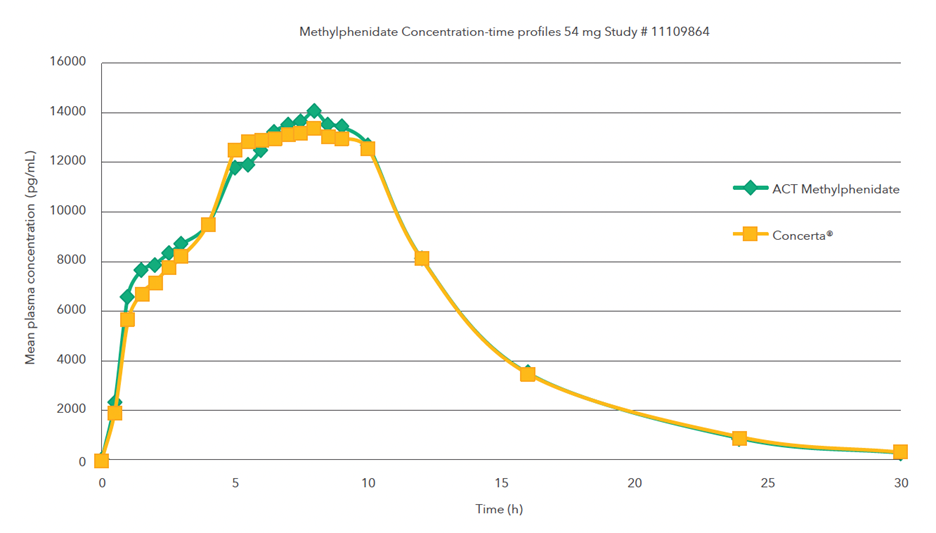
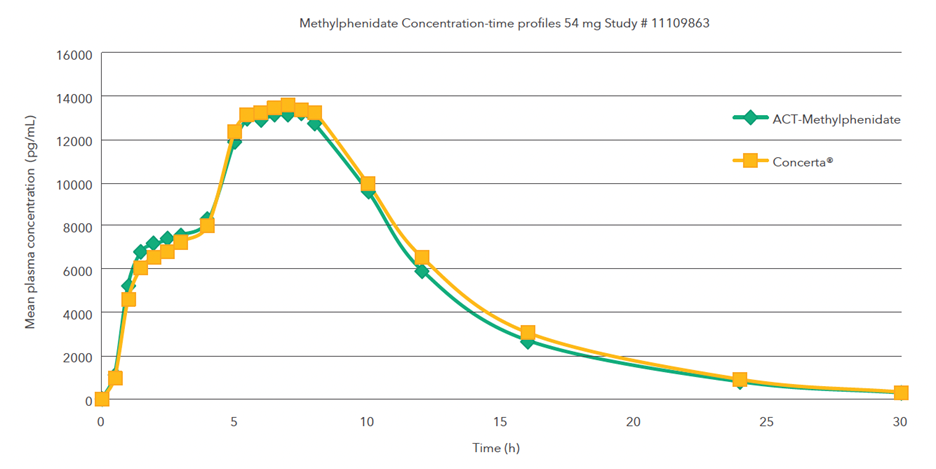
Single oral dose (1 x 54 mg) comparative bioavailability study under fasting conditions
Mean methylphenidate concentration-time profiles after administration of the test formulation (treatment A) and the reference product (treatment B)1,3,#
§A single-dose, two-period, two-treatment, two-way crossover bioequivalence study of methylphenidate hydrochloride 18 mg extended-release tablets (Teva Canada Limited) and Concerta® (methylphenidate hydrochloride) 54 mg extended-release tablets by Janssen Inc., Canada was conducted in healthy adult subjects under fed conditions.
#A single-dose, two-period, two-treatment, two-way crossover bioequivalence study of methylphenidate hydrochloride 18 mg extended-release tablets (Teva Canada Limited) and Concerta® (methylphenidate hydrochloride) 54 mg extended-release tablets by Janssen Inc., Canada was conducted in healthy adult subjects under fasting conditions.
References:
- Teva Canada Limited. ACT Methylphenidate ER Product Monograph. February 14, 2024.
- Data on File Study #3006968.
- Data on File Study #3006969.
- Data on file Study #11109864.
- Data on file Study #11109863.
Proven bioequivalence to Concerta®
The ACT Methylphenidate ER modified release dosage formulation has been developed to offer a comparable methylphenidate release profile to Concerta®.
Watch the comparative bioavailability video.
Learn more about ACT Methylphenidate ER



